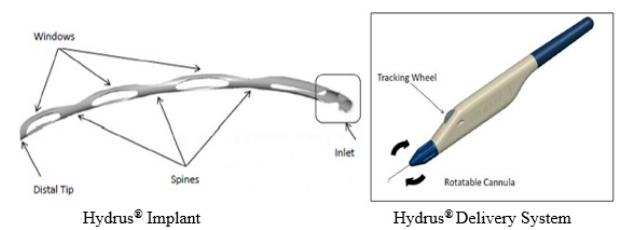
What is it? The Hydrus ® Microstent
It is a device which is implantable, flexible, a metal nitinol (Nickel Titanium) tube with windows (open-back stent) pre-loaded onto a hand-held delivery system which is used to implant the stent. The Hydrus® Microstent is meant to decrease eye pressure (intraocular pressure, or IOP) in grown-up individuals with moderate to modest primary open angle glaucoma (POAG) by functioning as a support structure in one part of the natural drainage path of the eye (Schlemm’s canal). POAG is a kind of glaucoma where
there is associated eye disease causing increased eye pressure and also where the eye pressure normally increases gradually. This progressive increase in eye pressure can be related to damages to the optic nerve which will impair vision significantly.
How does it work?
The Hydrus ® Microstent is implanted right into the eye of glaucoma patients to help fluid in the front part of the eye anterior chamber, or AC flow much more easily through Schlemm’s canal.
When is it used?
The Hydrus ® Microstent is intended to be used during cataract surgery. This is important for the reduction of intraocular pressure (IOP) in adult patients with mild to moderate POAG.
What will it accomplish?
In a medical research of 369 individuals, 77.2% who obtained the Hydrus ® Microstent attained a 20% or higher reduction in their IOP (intraocular pressure) compared to 57.8% (108/187) of the individuals having cataract surgery alone.
When should it not be used?
The Hydrus ® Microstent should not be used in patients who have any of the following problems:
When the colored part of the eye (iris) is pushed up against the drainage pathway (Schlemm’s canal) or when various other material obstructs the drainage pathway (Angle closure glaucoma);
Traumatic glaucoma, malignant glaucoma, or inflammation of the eye tissues (uvea);
Glaucoma related to the growth of abnormal blood vessels in the eye (neo-vascular); or
Visible birth abnormalities of the anterior chamber (AC) angle.